

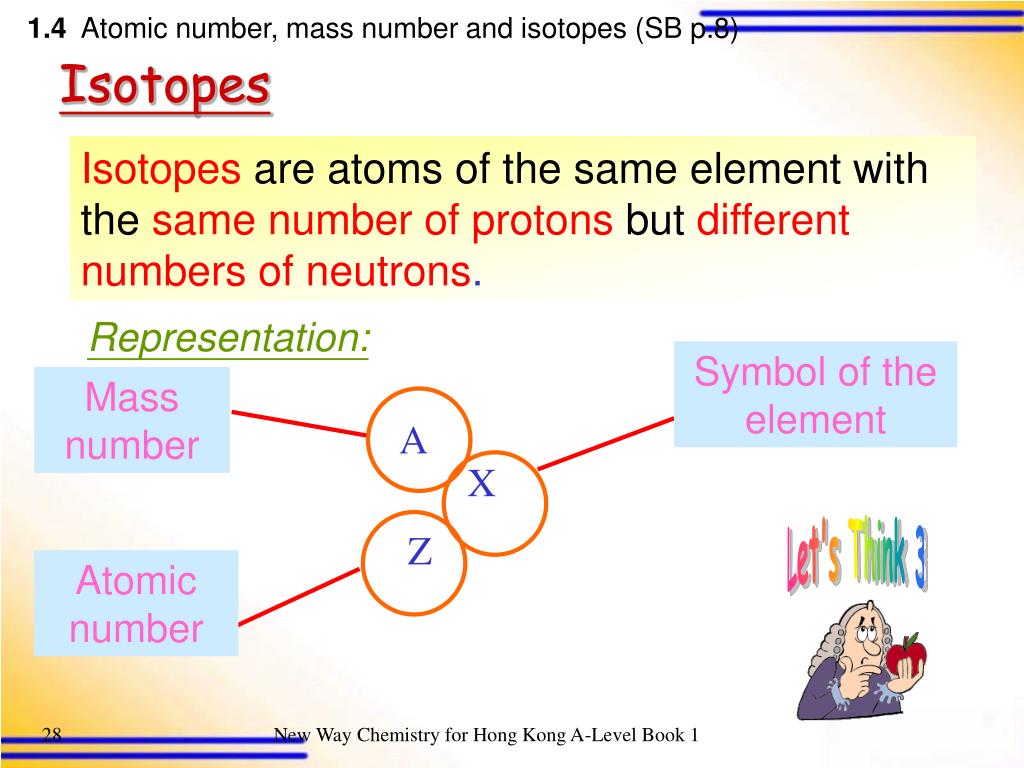
Two different elements always have a different atomic number as in 40 Two different elements can have the same mass number as in 40 This is true for any oxygen atom in the universe. It is always fixed for a given element, for example, the atomic number of an oxygen atom is 16. It can be different for a given element, for example, an oxygen atom can have mass number of 16, 17, or 18. Mass number is the number of nucleons (protons and neutrons) present in the nucleus of an atom.Ītomic number is the number of protons present in the nucleus of an atom. Mass number and atomic number are often confusing for beginners, but they both are different. Figure 1: Representation of Sodium-23 (Mass number is superscripted to the left of the symbol of sodium and atomic number, subscripted to left.) Mass Number and Atomic Number
DEFINITION ATOMIC MASS NUMBER FULL
When using the full name of an element, not its symbol, mass number come after the name of an element with a hyphen in between, for example, sodium-23, carbon-12, iron-56. The number is superscripted to the left of the symbol of sodium, which is Na (see the below figure). Consider an example of sodium, which has a mass number of 23. The mass number is represented as the left superscript to the symbol of an element. Where: n p + is the number of protons and n n 0 is the number of neutrons. The value of A is calculated by summing the number of protons and neutron in the nucleus of an atom. Mass number is also called as atomic number or nucleon number. Mass number is the number of nucleons (the sum of protons and neutrons) present in an atom. 5 Mass Number, Atomic Number and Neutron Number.Mass number is always a whole number because the number of protons and neutrons present in an atom is always a whole number. Since protons and neutrons are much heavier than electrons, the mass of an atom is estimated by the number of protons and the number of neutrons or in other words, mass number. Mass number, as from the name, is responsible for the mass of an atom or atomic mass. The number of nucleons present in an atom is called mass number. Protons and neutrons reside in the nucleus of an atom, and they, in together, are called nucleons. An atom consists of electrons, protons, and neutrons.


 0 kommentar(er)
0 kommentar(er)
